What Happens When the PHE Ends? Expanding Public Access to Community Pharmacy Services Beyond the COVID-19 Pandemic, Part 5
The following is part 5 of 6 in a series of articles titled, "Expanding Public Access to Community Pharmacy Services Beyond the COVID-19 Pandemic" by Jason Ausili, PharmD, MSLS, Head of Pharmacy Transformation for EnlivenHealth.
Improving Access to Care
Since the declaration of COVID-19 as a public health emergency on January 31, 2020, the federal government has made significant regulatory changes in response to an overwhelmed healthcare system and the need to improve access to desperately needed care:
These changes were brought to life through guidance documents provided by the Office of the Assistant Secretary of Health (OASH) and PREP Act Amendments aimed at mobilizing community pharmacists to expand COVID-19 testing, vaccination, and treatment services among the U.S. population. Their accessibility, convenience, training, education, skills, and experience make the nation's community pharmacists uniquely positioned to respond in the event of a PHE. Just as pharmacists played a key role in combating the 2009 swine flu pandemic caused by the H1N1 influenza virus, they have joined the battlefield once again with COVID-19 to help a nation in despair.
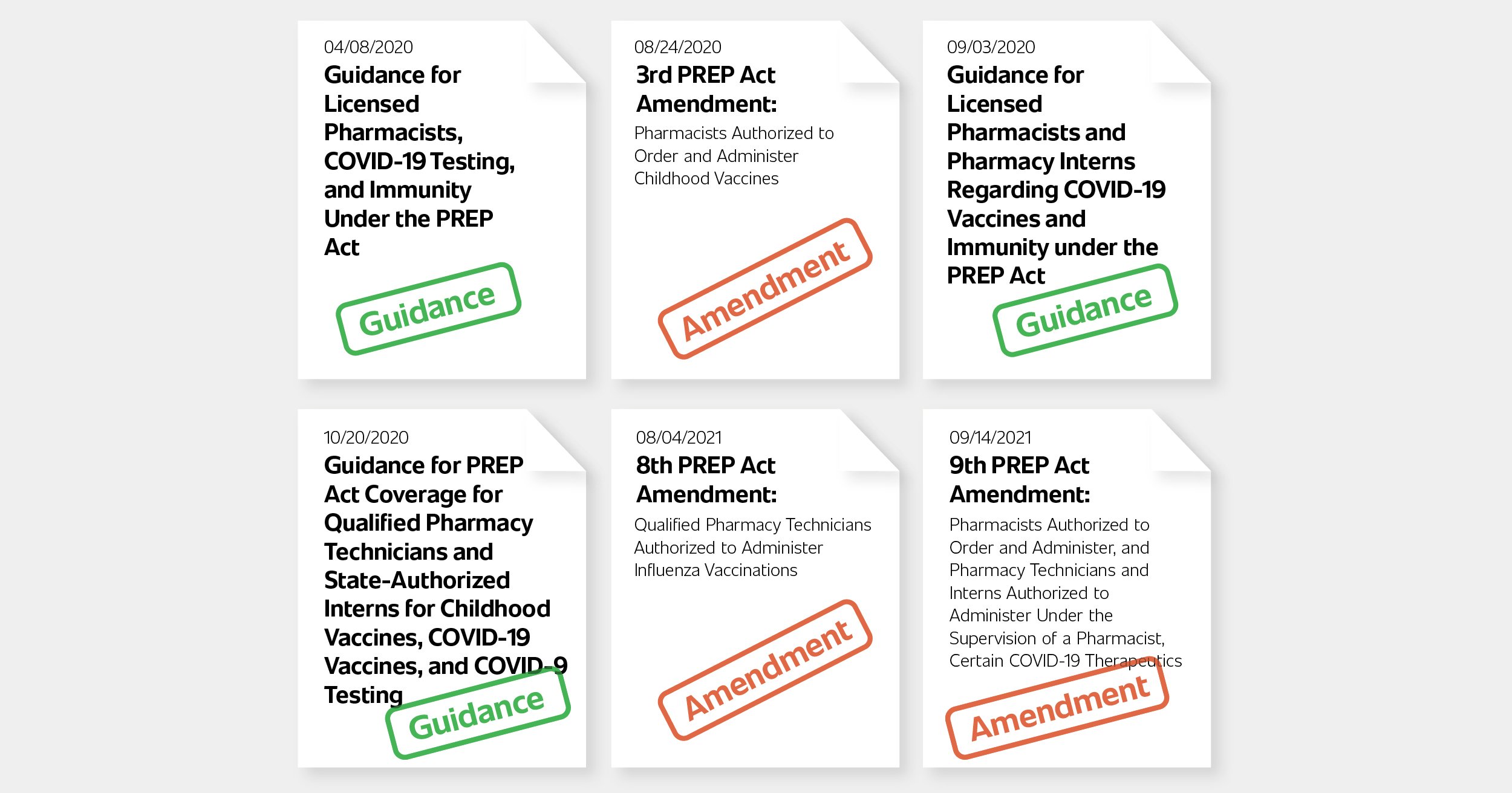
Although most states permitted pharmacists to perform POCT in conjunction with CLIA-waived tests prior to the pandemic, the HHS guidance released on April 8, 2020, authorized pharmacists nationally to order and administer COVID-19 tests that have received FDA EUA approval. This guidance was introduced at a time when vaccines were not yet available and the only countermeasures available to the public were face coverings, testing, and isolation upon receiving a positive test. COVID-19 tests were hard to come by during this period and the activation of the pharmacy channel helped more people get tested.
In response to a decreasing number of childhood vaccinations and a growing gap in vaccination schedules, the 3rd Amendment to the PREP Act authorized State-licensed pharmacists to order and administer (and pharmacy interns under the supervision of a State-licensed pharmacist to administer) all ACIP recommended vaccines to children three through 18 years old. Prior to this amendment, many states already had supportive regulation, but variances existed regarding age ranges and vaccines pharmacists were approved to order and administer. This provided a consistent and level playing field across the nation to address the growing childhood vaccination deficit.
Expanding Pharmacy Scope & Accessibility
On September 3, 2020, as COVID-19 vaccine development was getting closer to FDA EUA approval, the OASH provided guidance authorizing State-licensed pharmacists to order and administer (and pharmacy interns under the supervision of a licensed pharmacist to administer) COVID-19 vaccines authorized by the FDA to persons ages three and older.
Pharmacists have played an increasingly important role in vaccinating their communities during the pandemic as over 250 million vaccines have been administered at pharmacies across the nation. If this important channel was not available to the public, many people would have waited much longer, or possibly even had foregone the vaccine due to access and convenience issues.
The next important regulatory change brought about by the OASH expanded access to community pharmacy services by extending liability immunity and authorization of qualified pharmacy technicians and state-authorized pharmacy interns to administer FDA authorized COVID-19 vaccines, ACIP recommended vaccines, and COVID-19 tests. Detailed requirements were provided to qualify pharmacy technicians and pharmacy interns as "covered persons" and "qualified persons" under the PREP Act, including:
the stipulations that necessitate the vaccine must be ordered by a supervising qualified pharmacist
the supervising pharmacist must be readily available to qualified technicians
an ACPE-accredited practical training program must be completed
proof of a current certificate in basic cardiopulmonary resuscitation
the completion of a minimum of two hours of ACPE approve immunization-related continuing education
This expansion allowed community pharmacies to better scale vaccination operations in the face of high public demand through more qualified staff. As the country was approaching a potentially troublesome overlap of Flu season with the COVID-19 pandemic, the 8th Amendment to the PREP Act declared that qualified pharmacy technicians were authorized to administer influenza vaccines as well.
The Response to the COVID Variants
In late spring of 2021, the U.S. felt some relief with declining COVID-19 caseloads. This comfort came to a screeching halt in June of 2021 when the delta variant rapidly became the most prevalent version of COVID-19. Delta was more contagious than previous variants and caused more severe disease in the unvaccinated population, leading to large surges in the fall of 2021 even in the "most vaccinated" states.
With the looming Flu season ahead and a raging delta variant surge, conditions were optimal for community pharmacy to play a larger role in preventing influenza, which has a similar symptom profile. In parallel, COVID-19 therapeutics were becoming more relevant as advancements in monoclonal antibody therapies and pending oral antivirals were coming into view.
On September 14, 2021, the 9th Amendment to the PREP Act was announced, authorizing State-licensed pharmacists to order and administer (and pharmacy technicians and interns to administer under the supervision of a pharmacist) certain FDA authorized, approved, or licensed COVID-19 therapeutics. The declaration specified that the COVID-19 therapeutic must be ordered for subcutaneous, intramuscular, or oral administration routes "in accordance with FDA approval, authorization, or licensing."
The news of the 9th Amendment came shortly after the FDA's announcement of the EUA allowing the monoclonal antibody product, REGEN-COV, to be administered subcutaneously for post-exposure prophylaxis in those at elevated risk for progression to severe COVID-19 illness, including hospitalization or death. Immediately, community pharmacists took the helm to help save lives among the populations most vulnerable. CMS followed suit by providing a fee schedule, reimbursement mechanism, and published administration fee that covered the administration of four subcutaneous injections and a one-hour reaction observation period. Despite the early success of community-pharmacy based REGEN-COV administration in the fall and winter months, the FDA limited its use in January of 2022 due to its inefficacy against the omicron variant.
Short-Lived Privileges
Although these regulatory changes have had a dramatic effect on increasing access to community pharmacy-based services during COVID-19, the privileges granted may be short-lived. They are at considerable risk of ending with the expiration of the PHE, which the Biden Administration has to extend every 90 days. What will happen when the PHE expires and the public loses access to care that they once heavily depended upon? Will the dissolution of the current OASH guidance documents and PREP Act amendments lead to a devastating increase in health inequities?
The State of the Union address, the introduction of the ECAPS Act, and recent FDA authorization of pharmacists as prescribers of Paxlovid™ have procured hope that convenient access to community-pharmacy-based care is still a priority. But the AMA opposition to the pharmacy service expansion has challenged forward progress on this front, and in some cases has slowed or even blocked access to care. What if the ECAPS Act falls victim to the same opposing forces and fails to make it through Congress? Have we not learned from H1N1 and COVID-19 that when the country is in crisis mode, we need community pharmacists?
The privileges granted by the recent guidance documents and amendments must be made permanent for the nation to fully recover from this pandemic and to strengthen our defenses in preparation for the next one.
NEXT — Part 6: Building the Future of Healthcare »